"Soul Bargaining" has repeatedly staged centralized procurement, forcing China's pharmaceutical industry to add "original" quality
Our reporter Zhang Min, trainee reporter Guo Jichuan
On December 22, an investor asked the listed company Tonghua Dongbao: Has the company reassessed its profit forecast for the next two years?
Tonghua Dongbao, who participated in the sixth batch of national drug centralized procurement-insulin special, replied that he was confident to ensure that the company's revenue after centralized procurement showed a steady and increasing trend.
This is a microcosm of the pharmaceutical industry's face-to-face centralized procurement.
In 2021, the national drug centralized procurement policy will gradually shift from pilot to normal operation, reshaping the entire market structure: the intensively launched fourth, fifth, and sixth batches of centralized drug procurement will accelerate the squeeze out of false high prices.
At the same time, centralized procurement has accelerated the restructuring of China's pharmaceutical industry, increased the concentration of the generic drug industry, and increased the proportion of innovative drugs. Centralized procurement has formed a positive guide to the compliance, promotion model, capital investment and valuation judgment of the entire pharmaceutical industry.
With the continuous spur of centralized procurement, China's pharmaceutical industry is further accelerating the process of innovation and transformation. In this process, the wisdom of pharmaceutical companies is also tested.
"Life is not easy!" A person in charge of a pharmaceutical company once told reporters, "If you don't transition, you are waiting to die. There may be hope for transition."
Centralized procurement into normalization
Centralized procurement can be understood as a large-scale drug "group purchase", which clarifies the purchase volume, and realizes the price-for-volume exchange and lower the price of purchased drugs through market-based bidding among enterprises.
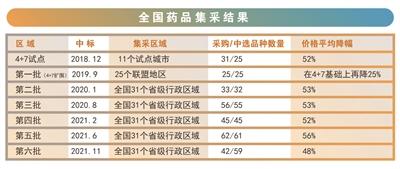
If the centralized procurement in 2018, 2019, and 2020 is a small test, then the centralized procurement in 2021 has entered normalization, and "soul bargaining" has been staged repeatedly.
In January of this year, the General Office of the State Council issued the "Opinions on Promoting the Normalized and Institutionalized Development of Centralized and Volumetric Procurement of Drugs", once again clarifying that centralized and volumetric procurement of drugs is an important measure to coordinate the advancement of the supply-side reform of medical services, and promote centralized drug procurement. The regularization and institutionalization of mass procurement will accelerate the formation of a unified and open national drug procurement market.
The issuance of the policy further supports the normalization of centralized procurement. In 2021, centralized procurement will gradually expand in multiple fields.
In February, the fourth batch of centralized procurement was launched. The average price of selected products was reduced by 52%, and the highest drop reached 96%. This round of centralized procurement was included in injections for the first time; in June, the fifth batch of centralized procurement was launched, and the scale of procurement reached a record high. Competition for injections It was particularly fierce. In the end, 61 drugs were successfully purchased, with an average price reduction of 56%. In November, the sixth batch of centralized procurement was launched. 42 products were selected, and the average price of the selected products was reduced by 48%, with a maximum reduction of 70%.
As the sixth batch of centralized procurement comes to an end in 2021, and the seventh batch of centralized procurement is ready to emerge in 2022, the industry expects that injections will once again become the "highlight".
At present, Qilu Pharmaceutical, Kelun Pharmaceutical, Yangzijiang Pharmaceutical, China Biopharmaceuticals, Hengrui Pharmaceuticals and other local pharmaceutical companies have passed the consistency evaluation in the number of injections. These companies have already geared up and are ready to meet the scale of 100 billion yuan. The injection market is shuffled.
In addition to the centralized procurement of medicines, the procurement of high-value consumables has also continued to advance.
On September 14, 2021, following the first procurement of coronary stents, the state organized the centralized procurement of artificial joints to open the bid in Tianjin. The price of the selected artificial joint products this time dropped from an average of 30,000 yuan to less than 10,000 yuan.
It is worth mentioning that with the continuous improvement of procurement models, systems and rules, centralized procurement is no longer "crossing the river by feeling the stones."
In this regard, the capital market reacted most sharply. The changes in the A-share pharmaceutical index clearly reflected the diminishing marginal impact of centralized procurement on the pharmaceutical sector.
When the results of the first “4+7” pilot centralized procurement were announced in 2018, the price of medicines fell by as much as 96%, which once triggered shock adjustments in the relevant sectors of the capital market.
In 2021, the results of the sixth special concentrated procurement of insulin were announced, and the price fell by as much as 70%, and the share prices of A-share related listed companies rose, because domestic pharmaceutical companies are expected to increase market share.
Chen Qiaoshan, a medical researcher at the Beijing Understanding Research Institute, told reporters that with the normalization of centralized procurement, pessimistic expectations have been released, the market's understanding of centralized procurement policies is deepening, and the mentality of investors has gradually returned to rationality.
Quality and quantity "supply" becomes the key
Winning the bid is only the first step for pharmaceutical companies to participate in centralized procurement, and quality and quantity "supply" is the key to the implementation of centralized procurement.
In 2021, supply cuts will appear many times, testing the integrity and strength of pharmaceutical companies and whether the centralized procurement system can proceed normally.
On August 20, Shanghai Sunshine Pharmaceutical Purchasing Network issued the "Announcement on Including North China Pharmaceutical Co., Ltd." on the Violation List, stating that North China Pharmaceuticals, the selected company for the third batch of centralized procurement of ibuprofen sustained-release capsules, is located in Shandong Province Failed to supply the agreed procurement volume in accordance with the agreement. After many interviews and consultations, it still did not improve. It was decided to include it on the "violation list" and cancel its eligibility to declare for national centralized procurement before May 10, 2022.
On September 18, the Henan Provincial Public Resources Trading Center notified that the entecavir tablets of Beijing Biopharmaceuticals were selected as the selected species in the national centralized procurement pilot expansion. There were many supply problems in Henan Province, such as low distribution rates, from May to June 2021. In August, there were problems such as production suspension and supply cut.
According to relevant documents and regulations, the price of medicine and the credit rating of Baiao Pharmaceuticals in Henan Province were rated as "serious".
On November 8, the Hubei Provincial Medical Security Bureau issued a notice stating that Sandoz (China) Pharmaceutical Co., Ltd., the successful bidder product of Losartan Potassium Tablets (50mg), could not be supplied normally and would be supplied by Huahai Pharmaceutical as a substitute.
There are many reasons for the interruption of supply, including the transformation of production lines and the rising cost of raw materials. Is it due to insufficient production capacity or limited profits?
This has triggered heated discussions in the industry.
With the continuous advancement of centralized procurement, the types of centralized procurement of drugs and high-value consumables continue to increase, the competition among pharmaceutical companies is intensifying, and the industry is accelerating into an era of meager profit.
A person in charge of a pharmaceutical company who did not want to be named told the "Securities Daily" reporter, "The era of generic drugs lying down to make money is over, and profit margins have been greatly compressed. Not participating in centralized procurement means losing the market, and participating in centralized procurement means reducing gross income. Interest rates and small profits are the general trend."
Behind the cut-off of supply by many selected pharmaceutical companies, the industry has also raised concerns about "low-priced drugs".
"Bulk purchases were launched before, but the result was that some drugs disappeared from the market after price cuts." An industry insider told reporters that from the trend of centralized procurement, price cuts have become moderate, and policies are also being adjusted. .
On September 29, 2021, the General Office of the State Council issued the "Notice on Printing and Distributing the "14th Five-Year" National Medical Security Plan", stating that by 2025, each province (autonomous region, municipality directly under the Central Government) will purchase 500 varieties of drugs in a centralized manner at the national and provincial levels. Above; centralized procurement of medical consumables reaches more than 5 categories.
This also means that in the next few years, national centralized procurement will still be the "big test" facing the pharmaceutical industry.
The general trend of innovative drug development
In the context of centralized procurement, the profit margin of pharmaceutical companies has been reduced.
Hengrui Pharmaceuticals released its third quarterly report showing that in the third quarter it achieved operating income of 6.9 billion yuan, a year-on-year decrease of 14.84%; realized net profit attributable to shareholders of listed companies of 1.54 billion yuan, a year-on-year decrease of 3.57%.
Although the reasons for the decline in performance were not disclosed in the third quarterly report, Hengrui Medicine had previously stated in the 2021 semi-annual report that the company’s third batch of centralized procurement started in November 2020 involved 6 drugs, which caused the company to go up this year. Half-year sales revenue fell 57% month-on-month.
On August 30, MicroPort Medical, a Hong Kong-listed company, issued an announcement showing that in the first half of 2021, the company lost approximately US$115 million.
One of the reasons is that the national centralized procurement policy of coronary stents has brought about a significant increase in the sales of cardiovascular intervention products, but it has not fully compensated for the decline in revenue and gross profit at the same time.
The state squeezes out the price of generic drugs and high-value consumables through centralized procurement, and at the same time encourages pharmaceutical companies to invest heavily in the development of innovative drugs with clinical value.
In the first three quarters of this year, there were 6 companies that invested more than one billion yuan in R&D in the A-share pharmaceutical sector, and the number of companies that invested more than 100 million yuan in R&D accounted for about one-third of the total.
With the deepening of centralized procurement, it is an inevitable trend for pharmaceutical companies to shift their energy to innovative drugs.
Yu Zilong, the managing partner of KPMG China’s life sciences industry, told the Securities Daily reporter that in an environment that encourages pharmaceutical innovation, innovative drugs are used to form differentiated competitive advantages, drive sales growth, and obtain higher profits. The transformation direction of the enterprise.
Zhang Lichao, senior researcher at Guosen Securities, told reporters that the core competitive advantage of pharmaceutical companies in the future will come from the thickness of the R&D pipeline and the ability to control costs.
The localization rate is the key to promoting various medical cost reduction measures in the future. On this basis, policy optimization, product innovation, transformation and upgrading, and local advantages will be the main investment logic of the pharmaceutical sector.
Wang Lun, chief investment consultant of Shenzhen Newland, said, “Innovation and leadership are becoming the two major trends in the future pharmaceutical industry, and they are also the directions that investors need to pay attention to. From a policy perspective, it is necessary to observe whether the supporting policies for the innovative drug industry are With continuity, this is an important support for future innovative drugs to generate returns."
Pharmaceutical stock investment logic reversed
Facing the normalization of centralized procurement, the investment logic of the pharmaceutical sector has undergone significant changes compared with the past, which is manifested in the readjustment and re-understanding of sector valuation.
The market enthusiasm of the pharmaceutical sector and the focus of investors' attention are shifting in the direction of pharmaceutical innovation and industrial upgrading.
The old era of relying on medicines to support medical care and sales has passed, and technological innovation capabilities are becoming an important criterion for measuring the value and growth of pharmaceutical companies.
Gong Tao, chairman of Shenzhen Zhongjin Huachuang Fund, told reporters that centralized procurement is a double-edged sword. On the one hand, it provides more market space for domestic pharmaceutical companies; Price pressure has promoted the transformation of pharmaceutical companies with serious product homogeneity.
Judging from the listing of innovative pharmaceutical companies, since 2018, 77 biopharmaceutical companies have landed in Hong Kong stocks to raise funds.
As of December 22, 2021, 75 biomedical companies have been listed on the Science and Technology Innovation Board.
On December 15, BeiGene, which continued to invest in research and development and was still at a loss stage, officially listed on the Sci-tech Innovation Board, becoming the first biopharmaceutical company listed in three places (Nasdaq, Hong Kong Stock Exchange, and Shanghai Stock Exchange).
From the perspective of product layout, pharmaceutical companies continue to increase their investment in innovative products and gradually form their own product portfolio advantages.
Yu Zilong believes that the main investment opportunities in the pharmaceutical sector in the future will focus on the following aspects, including pharmaceutical companies that have a certain scale and cost advantage in specific treatment fields, new pharmaceutical retail and digital promotion service platforms, and academic promotion capabilities. Sales outsourcing service pharmaceutical companies, biopharmaceutical companies with real innovation capabilities, etc.
At the same time, pharmaceutical companies that rely on generic drugs and original drugs with expired patents will gradually decline.
Zhang Cuixia, chief investment consultant of Jufeng Investment, told the "Securities Daily" reporter that more and more pharmaceutical companies are reducing their reliance on generic pharmaceutical products by devoting themselves to pharmaceutical R&D and medical services, and the investment trend of the pharmaceutical sector is also more diversified.
In addition to innovative drugs and innovative medical devices, leading companies that are sought after by the market have emerged in biomedicine segments such as off-hospital pharmacies, Internet medical services, third-party laboratories, and vaccines.
After many rounds of centralized procurement, China's pharmaceutical industry is emerging from the "acceleration" of innovation and development.
(Securities Daily)