Saturated Nurse This is how the new antigen tests for flu and Covid work
Boticaria García Back to school with an antigen test in the pharmacy capable of detecting Covid and influenza A and B
"We have a simple message for all countries: test, test, test."
Almost three years have passed since Tedros Adhanom, the Director General of the WHO, uttered these words.
In this time the circumstances have changed significantly but
the tests continue to be a key tool when it comes to controlling the pandemic
, but not only: they are also very useful in the detection of influenza A and B, thanks to the tests that combine the Covid with these other pathologies.
This utility is seen especially at peak times like the one we are experiencing right now with the flu, an absolutely unusual outbreak in other seasons.
The reason: Because we haven't been exposed to these viruses during the pandemic, this winter brought forward the outbreak, which normally begins in November and December (and peaks in January).
In that case it was influenza A, but those that are now being registered in the consultations are influenza B, according to the latest report from the
Acute Respiratory Infection Surveillance System (Sivira)
, prepared by the Carlos III Health Institute (ISCIII).
In the corresponding to week 5 of this year (from January 30 to February 5),
The flu positivity rate registered in primary care had increased for the third consecutive week, standing at 24.8%
, with the highest rates
among those under 15 years of age.
When the WHO appealed to the tests in those early moments, the tests were still
designed exclusively for professional use
, as is the case with most diagnostic tests.
It was necessary to have professionals both for the taking of samples and for their analysis.
In addition, in times of shortage of everything (masks, PPE, respirators... and also tests) the doctors were the ones who had to decide in which cases to perform it.
It would take
more than a year for these same tests to arrive
, in a domestic format,
at Spanish pharmacies
.
The aim was both to provide some relief from a saturated and exhausted healthcare system and to offer citizens the possibility of
obtaining their own diagnosis without having to go to the doctor
.
All this as part of a global strategy to control the virus.
For this to be possible, it was necessary, first of all, to have a type of test that could be handled by the ordinary citizen.
The labs had worked on
a fast and reliable version
for both the antigen test (tracks for active infection) and the antibody test (deals with whether infection has been passed in the past).
The taking of samples had been simplified and the mechanism of the test was as simple as that of another old acquaintance: the pregnancy test.
Navidad Sánchez Marcos, member of scientific production of the Spanish Society of Clinical, Family and Community Pharmacy (Sefac), explains that these tests are based on the same technique: scanning immunochromatography, "which allows the determination of the presence or
absence
of of a substance in a sample and is the technique of choice in self-diagnosis tests due to its simplicity and speed in reading the results (qualitative yes/no), as well as its reliability (sensitivity and specificity)".
"Unlike products for exclusive use by professionals," says Daniel Fernández Font, head of the Parapharmacy Area of the General Council of Pharmaceutical Colleges, "all self-diagnosis products are certified by a notified (and independent) body that evaluates all the documentation submitted by the manufacturer and determines that the test is suitable for use by the general population".
With a test specifically designed for non-professional use, there was still another stumbling block:
Spanish legislation did not allow the sale of this type of test
, so an exception had to be introduced so that citizens could not only purchase them in pharmacies, but also Furthermore, they did so
without the need for a medical prescription
.
The Ministry of Health recalls that it is a temporary exception subject to exceptional circumstances
, although it is not within its plans to withdraw it in the short term since "currently Covid-19 continues to represent a Public Health problem."
So at the moment Royal Decree 588/2021, of July 20, approved to modify Royal Decree 1662/2000, of September 29, on medical devices for in vitro diagnosis, will not be
modified
.
However, at this point, tests are part of our 'new normal' and we have become so used to them that it would probably be difficult for us to understand that such access would be limited in the future.
The person in charge of Parapharmacy of the General Council of Pharmaceutical Colleges advocates
maintaining this exception
: "Covid-19 has come to stay and we are going to live with this pathology, as one more among the different respiratory viruses in our environment. The self-diagnosis tests against to Covid-19 are one more diagnostic tool, which can help the population find out if the symptoms they have are caused by Covid and take the appropriate measures in the face of possible social interactions, especially contacts with vulnerable people in their environment.
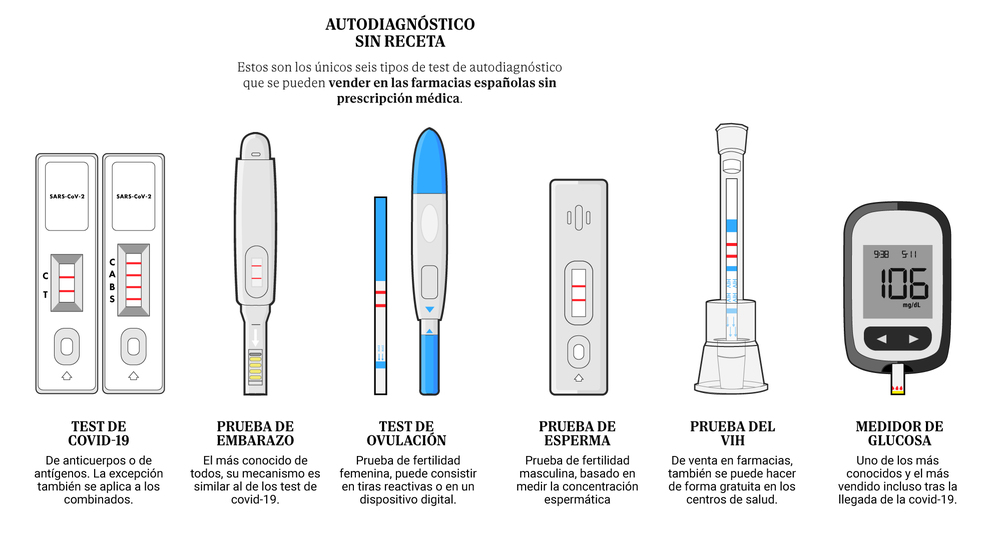
The other self-diagnostic tests
There are other (few) exceptions
to the impediment to sell (and advertise) self-diagnosis products such as Covid-19.
Added to the pregnancy test are the male and female fertility tests, self-diagnostic products for the determination of blood glucose and those for the detection of HIV.
All of them can be sold without a prescription.
The Ministry of Health justifies these exceptions based on "Public Health criteria (HIV, Covid), monitoring of chronic diseases (glucose levels in diabetics) or when the intervention of a health professional is not considered necessary, who prescribes and interprets the results obtained, establishing a diagnosis and advising the patient".
The HIV test was the last to be added to this list before the pandemic
, at the end of 2017. It is still possible to get tested for free at the Public Health, but this possibility was added to help early diagnosis and to try to reduce the number of people living with the infection without knowing it (in 2021 it was estimated that there could be about 20,000).
But there are
many other tests that have a 'domestic' version and that are not part of this exception
.
"Currently, there are around 20 different determinations that can be carried out with the self-diagnosis tests that are dispensed in pharmacies," says the person in charge of Parapharmacy of the General Council of Pharmaceutical Colleges.
"Among these tests, the detection of certain urinary tract infections
, the determination of
prostate specific antigen (PSA) levels
,
cholesterol
or the determination of
vitamin D levels
can be performed
."
This type of test
is not in excessive demand
, which is logical since they cannot be advertised and are subject to a prescription.
"They are greatly underused," says the Sefac member.
"From our point of view, it would be preferable if they were tests that could be performed from community pharmacies as one more service, allowing access to this type of test (celiac disease, helicobacter pylori, iron deficiency, multidrugs, HIV, urinary tract
infection
, oropharyngeal infection, etc.)".
For experts it makes more sense to extend the exception than to make it restrictive again
.
And the numbers do not lie, according to data from the consultancy Iqvia, more than 112 million coronavirus tests were sold during the year 2022, which represented 33% of the total sales of self-diagnosis tests in our country.
In the words of Fernández Font, "we know that this type of test is already part of many people's medicine cabinets and is used when they have symptoms compatible with Covid-19 and a trip, a family reunion, etc. is planned."
The truth is that for now, far from withdrawing this exception, we have seen how
the Covid-19 test was combined with that of other better-known pathologies
: the
flu
and the respiratory syncytial virus (
RSV
, the culprit of the bronchiolitis that took over the emergencies at the end of last year).
Rapid flu diagnostic tests have been around for years, but for professional use.
They are designed to identify the presence of
influenza A and B virus
antigens , differentiating them or not in the results, but they do not have great sensitivity (50-70% probability of not obtaining a false negative).
There is, however, no 'self-diagnosis' version in the registry of the Spanish Agency for Medicines and Health Products (AEMPS), at least not without being combined with Covid-19, thanks to which they are indirectly included
within the exception introduced for the sale of the latter
.
"Although the tests for Covid-19 that simultaneously detect other antigens are not expressly mentioned, given the presence of these combined tests on the market, it is
understood and interpreted as included in this legislation
, since the objective of this regulation was to increase the capacity diagnosis during the health crisis, which has not yet ended. In this way, the combined tests would be excluded from the need for a prescription," they explain from Health.
There are currently 71 rapid Covid-19 antigen tests marketed in Spain, and only 6 of the combined type, of which only two include RSV (one from a Chinese manufacturer and another from the United States).
Regulation in other European countries
What happens in other countries?
" The regulation of
in vitro
diagnostic products
is common at the European level
", they explain from the Ministry of Health, "these products are regulated in Europe by Regulation EU 2017/746 of the European Parliament and of the Council of April 5, 2017 on
in vitro
diagnostic medical devices
, which entered into force on May 26, 2022 and establishes transitional periods for different products, depending on their classification".
The regulation reflects in section 9 of its Article 1 that it will be applied "without prejudice to national law relating to the organization, provision or financing of health services and medical care".
This means that
each country decides on the points of sale allowed or on whether it is necessary to have a medical prescription
.
For this reason, it is possible that in some neighboring countries these and other self-diagnostic tests are sold in supermarkets.
"In the vast majority of the countries around us," says Fernández Font, "this type of product is without a prescription and advertising can be done."
From Roche Diagnostics they affirm that "in other European countries it is easy to find private practices in which rapid self-diagnosis tests can be carried out, as long as the doctors/specialists that compose them consider it appropriate, and depending on the test, it is usually reimbursed by the system itself health of the country".
Our legislation is somewhat more restrictive, but the obligation to go through a pharmacy does not seem like an unjustified requirement
.
"We believe that it is important that there is a health professional behind it, either a doctor who prescribes it or a pharmacist who indicates it, since resources must be optimized. It also does not make sense that tests are carried out in situations and patients without need, for the simple reason for being freely available to the public", they say from Sefac.
Pharmacists carry out a task that goes beyond selling the test in question, they offer
necessary information
for its correct use and for its interpretation, in addition to indicating the appropriate measures given the result obtained.
They also
monitor
the patient, which is essential when faced with sensitive tests such as HIV.
And it is precisely the pharmacists who defend that this type of test be established definitively and without a prescription
.
"We believe that tools that allow the early detection of diseases should be available in the pharmacy," they say from Sefac.
"In addition, we believe that this type of test should continue to be freely accessible or under the professional criteria of the community pharmacist; that is, pharmaceutical indication, as is the case with medicines not subject to medical prescription."
In addition to better access to early detection, from the General Council of Pharmaceutical Colleges they also talk about "empowering the patient in the knowledge of their state of health".
The pandemic has caused many twists in the script, and perhaps this is for the better: has the time for self-diagnosis tests come?
According to the criteria of The Trust Project
Know more
Infectious diseases
Flu
coronavirus
Covid 19
Respiratory diseases