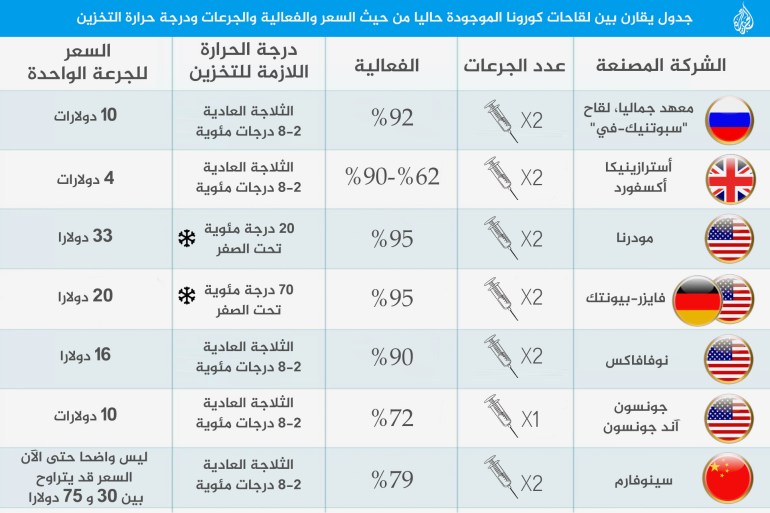
During the last period, data and recommendations appeared about the possibility of mixing vaccines for the emerging coronavirus "Covid-19", so what are the latest recommendations related to this?
By mixing vaccines, we mean giving the first dose of a specific vaccine, for example, "Pfizer-BioNTech", and then giving the second dose of another vaccine, such as the "AstraZeneca-Oxford" vaccine.
Why think about mixing corona vaccines?
Some countries face a shortage of vaccine supplies, so they will make up for the shortage by giving the available vaccine.
In Europe and Canada, millions of people there received an initial dose of the “AstraZeneca-Oxford” vaccine before governments recommended that younger age groups avoid it due to the risk of developing a rare clotting disorder, according to a report in the “Science” website.
Spain has allowed the mixing of two vaccines for people under the age of 60.
Other countries have set age limits for the AstraZeneca-Oxford vaccine, including Canada, Germany, France, Norway and Denmark.
And now we will review the latest recommendations and data about mixing Corona vaccines
1- The World Health Organization warns
Soumya Swaminathan, chief scientist at the World Health Organization, warned individuals against combining Covid-19 vaccines produced by different companies, describing this trend as "dangerous" due to the lack of much data on the impact on health.
“It is a very dangerous trend,” Swaminathan said, during an online briefing on July 12, and continued, “The situation will be chaotic in countries if citizens start deciding when to take a second, third and fourth dose, and who receives it.”
Swaminathan described the combination of different vaccines as "no data", but the World Health Organization clarified the next day (July 13) that some data was available, and more was expected.
The results of a clinical trial conducted by Oxford University on the combination of AstraZeneca, Pfizer, Moderna and Novovax vaccines are currently awaiting publication.
"Data on compatibility and combination studies are awaited, and there is a need to assess the immunogenicity and safety aspects," the World Health Organization said.
It added that public health agencies should make these decisions based on available data, not individuals.
2- Pfizer-Biontech vaccine can be used as a second dose
The World Health Organization's Strategic Advisory Group of Experts on Vaccines said last June that the Pfizer-Biontech vaccine could be used as a second dose after a first dose of AstraZeneca-Oxford if the latter was not available.
3- Strong antibody response
A Spanish study found that giving a first dose of AstraZeneca-Oxford vaccine and then a second dose of Pfizer-Bionic vaccine after 8 weeks;
It led to few side effects and a strong antibody response two weeks after the second dose, and the study was published in The Lancet as a "preprint" paper, meaning it hasn't been reviewed by other scientists yet.
4- T cells
Researcher Leif-Eric Sander, an infectious disease expert at the Charité University Hospital in Berlin, and colleagues found that giving a first dose of the AstraZeneca-Oxford vaccine and then a second dose of the Pfizer-Biontech vaccine 10 to 12 weeks later produced antibodies at levels similar to the group that received two doses of the vaccine. Pfizer-Biotech over a 3-week period, there was no increase in side effects.
And the T cells, which can boost the antibody response and also help rid the body of already infected cells, responded slightly better than the two Pfizer-Biontech recipients.
5- Much more side effects
Matthew Snape, a vaccine expert at the University of Oxford, and his colleagues study 8 vaccine variations in nearly 100 people each with a first dose of either AstraZeneca or Pfizer, followed by a dose of the same vaccine or vice versa, at an interval of 4 or 12 weeks.
A group of experts reported in The Lancet that people who received the Pfizer-Biontech vaccine just 4 weeks after the AstraZeneca vaccine experienced significantly more side effects than those who received two doses of the same vaccine, according to a Science report.
6- Confronting new breeds
According to a report published on the Gavi Vaccine Alliance website, by Praia Joy, mixing vaccine products may be a good idea for many reasons, as supply bottlenecks have led to shortages in many countries, so the ability to mix vaccines from different manufacturers may reduce pressure on the vaccine supply.
Furthermore, there is some preliminary evidence to suggest that it can also lead to a stronger immune response compared to two doses of the same vaccine.
Some countries are planning to mix vaccines due to shortages in supplies or concerns about rare side effects of some products.
The writer said that in addition to the potential positive effect on the immune system, mixing vaccines may also help prevent vaccines from becoming less effective in the face of new strains of the Corona virus.
As the virus mutates, the part of the vaccine that the vaccine targets can change, which could make the vaccine less effective.
But if there are two vaccines that target different parts of the virus, that gives our immune system more than one weapon in its arsenal.
The writer said that earlier this year 2021, AstraZeneca vaccine makers considered combining a first dose of their vaccine with a second dose of the Russian “Sputnik-V” vaccine.
Both vaccines use adenoviruses as a delivery system to deliver the coronavirus antigen (the scientific name for SARS-CoV-2) into our bodies and cells. By doing so, our immune system can build up an immunity not only against COVID-19, but also respond to the adenovirus that is being used as a vehicle.
This means that after a second dose of the same vaccine, our bodies may have antibodies against the adenovirus component that can neutralize the vaccine, making the second injection less effective.