Researchers from the Wuhan Institute of Virology in China have published the genetic sequence of 8 new coronaviruses from bats taken from an abandoned mine in Mujiang, Yunnan Province, so what does this mean?
What is the latest scientific data about vaccines and the emerging corona virus "Covid-19?" And how effective is it against the Indian strain? Answers and more are in this report.
The French newspaper Le Monde said that researchers published data on the Corona virus (whose scientific name is SARS-Cove 2) last Friday, indicating that the relevant scientific community will examine these new data in the coming days.
Virologist Etienne Decrouli, a researcher in architecture and biology functions at the Macromolecular Laboratory of the French National Center for Scientific Research and Aix-Marcy University commented, "What they have documented here is a strain between the SARS-Cove 1 virus responsible for the severe acute respiratory syndrome (SARS) 2003 epidemic and SARS-Cove 2" .
"Researchers of the Wuhan Institute of Virology said in their publication that the eight viruses in this new strain do not show affinity with the ACE2 receptor (human angiotensin-converting enzyme), and this means that the direct transmission of these viruses from bats to humans is something," DeCrule added. It is highly unlikely, and therefore they returned to the hypothesis of recombination between the bat corona virus and the pangolins corona virus, which has a strong link with ACE2 receptors in human cells. "
The researchers said that samples should be taken from bats and an anteater, or other potential intermediate animals, to better understand the origin of the Corona virus.
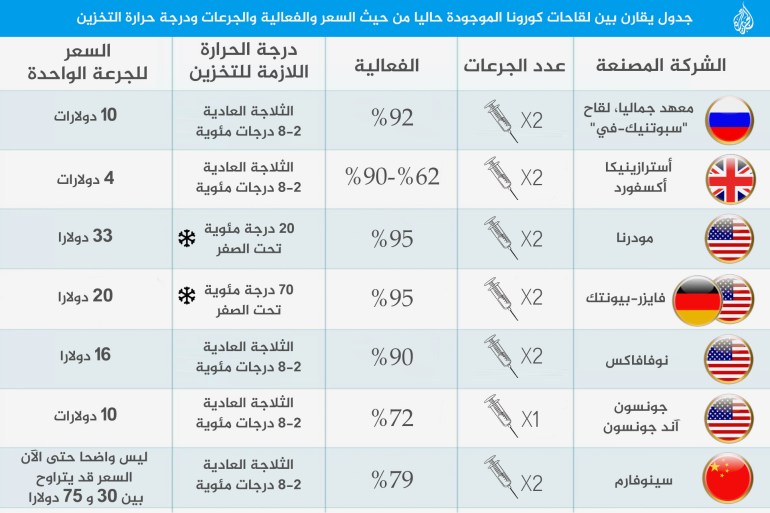
The Pfizer and AstraZeneca vaccines are effective against the Indian strain
A study conducted by the health authorities in England, and the results of which were published yesterday, Saturday, showed that the vaccines "Pfizer BioNTech" and "Oxford-AstraZeneca" anti-Coronavirus are effective against the mutated Indian version of this virus almost as effective as they are. Against the British version of it.
According to the study by Public Health England - between April 5 and May 16 - the Pfizer-Bionic vaccine, two weeks after receiving the second dose, provided 88% effectiveness against the symptomatic Indian strain, and 93% against the British strain. Accompanied by symptoms.
In contrast, the effectiveness of the AstraZeneca-Oxford vaccine was - two weeks after receiving the second dose - 60% against the symptomatic Indian strain, and 66% against the British symptomatic strain.
British Health Minister Matt Hancock welcomed the results of this study, which comes at a time when the government is counting on the national vaccination campaign to combat the Indian strain, whose outbreak threatens to derail the plan to reopen the economy in the country.
To limit the spread of this mutant, dubbed "B1 617 2" (B.1.617.2), which is feared to become "prevalent" in Britain, health authorities have reduced the interval between two doses of the AstraZeneca vaccine from 3 months to 8 weeks for people who overdose. They are over 50 and for those classified as the most vulnerable in terms of health.
These measures were also accompanied by the intensification of examinations aimed at detecting HIV infections in the most vulnerable regions, especially northwest England and some parts of London.
According to the study, the Pfizer-Bionic and AstraZeneca-Oxford vaccines were saved 3 weeks after receiving the first dose, with an efficacy of 33% against the symptomatic Indian strain, and by 50% against the British strain with symptoms.
According to data from the Public Health England Agency, at least 2,889 infections with the Indian strain were recorded in England between February 1 and May 18.
104 of these patients had to receive first aid in hospital emergency departments, while 31 of them stayed in the hospital and 6 died. "Two doses of either of these vaccines provide high levels of protection against the symptoms associated with infection with the Indian strain," said Miri Ramzai, who is in charge of the vaccination at the Public Health Agency. ".
A vaccination campaign after a "worrisome" mutated version of Corona was detected
All adult residents in a neighborhood of the city of Bordeaux, southwest of France, will be able to receive the vaccine quickly and unconditionally, after monitoring a focus of dozens of people who tested positive for a "worrisome" mutated version of the Corona virus, according to what the French Press Agency reported on Saturday.
At a time when the intensive examination of residents began Friday in the Bacalan neighborhood in the north of the city, Patrick Dohill, a medical and scientific advisor to the local health authorities, explained that they had requested to "unconditionally bring forward the date of vaccination of people over the age of 18 years" in this neighborhood and neighboring areas, so that it would take place at the weekend. If possible or at worst the beginning of next week. "
Dühl added that the goal after that is to expand the vaccination campaign to include "the city and its suburbs."
"This mutated version has already been observed in the country, but it is very rare so far," he said, "and it was also detected in the Parisian region of Ile-de-France."
The medical advisor added, "But it has not appeared much at the national or international levels so far. In principle, no foci of this kind have been recorded among the general population."
The number of infected group members is at least 46, without counting the results of the first day of the comprehensive examination that was launched on Friday.
None of the injured were vaccinated and "none of them were taken to the hospital, and they showed the usual symptoms or no symptoms," according to the medical official.
He pointed out, "There is no reason to believe that these infections will be more serious and that this mutant is resistant to (high-tech) messenger RNA vaccines," such as Pfizer or Moderna.
For its part, the French Public Health Agency indicated, in a statement on Friday evening, that this mutant "is still very rare at the international level and in France, despite the recent monitoring of several transmission chains in Bordeaux."
The mutant was named "VOC 20I / 484Q" (VOC 20I / 484Q) and it is derived from the mutant that appeared in Britain, but acquired an additional mutation (ie 484Q) suspected to reduce the effectiveness of vaccines. This mutation was recorded in the mutant that appeared in both South Africa and Brazil.