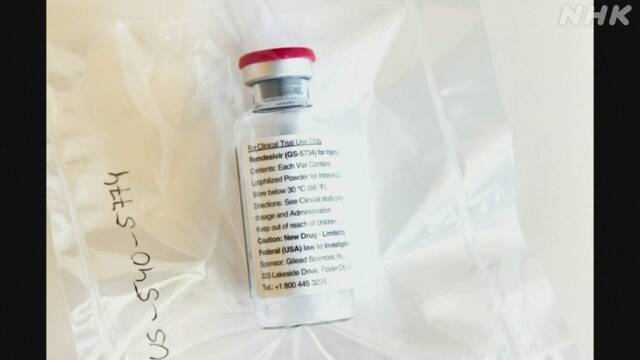
The United States officially approves the new corona remedy for "remdesivir" October 23, 11:37
As a therapeutic drug for the new coronavirus, the antiviral drug "remdesivir", which has been specially approved in Japan, has been officially approved in the United States.
The antiviral drug "remdesivir", which has been developed by an American pharmaceutical company as a therapeutic drug for Ebola hemorrhagic fever, is undergoing clinical trials as a therapeutic drug for the new coronavirus, and the US FDA = Food and Drug Administration has decided. In May, it was approved for emergency use, and in response to this, Japan also approved its use as a special case.
The FDA announced on the 22nd that it has officially approved "remdesivir" as a treatment for the new coronavirus based on the results of multiple clinical trials.
The target patients are 12 years old or older and need to be hospitalized with a weight of 40 kg or more.
Regarding remdesivir, in clinical trials conducted by the National Institutes of Health (NIH) in the United States, it was reported that it had the effect of shortening the recovery period of patients, and it was also administered to President Trump infected with the new coronavirus. ..
On the other hand, according to the results of clinical trials published by WHO = World Health Organization, it was evaluated that "almost no effect or no effect was observed" for improving patient mortality rate and shortening hospital stay. Is divided.