In 2020, the Nobel Committee awarded the Nobel Prize in Chemistry to Emanuela Carpenter and Jennifer Doudna for their efforts in the discovery and development of the famous CRISPR–Cas9 system, better known as CRISPR. and published in the journal Science in 2012.
Although CRISPR is not the first technology to edit genetic material, it has made the process of modifying genetic material a simple and affordable process. It is also a general technology that works with simple genomes such as the genome of bacteria, and complex genomes such as the genomes of plants, vertebrates and humans.
The technology of editing single genetic bases replaces one nitrogenous base with another (Getty Images)
Genome editing by CRISPR technology
The term "genome editing" means modifying the sequence of the genetic material of an organism, and the genetic material usually consists of a sequence of "nucleic acids" (DNA) that make up the genome of the organism that includes all its genes and intergenic regions.
In the event that there is a defect in one of the genes, for example, or the desire to transfer a gene from one organism to another organism - such as transferring the gene for insulin production from a human to a bacterium - a “modification” is made in the genome of the organism by cutting a specific point in the genome and then pasting the new transferred sequence. from the other object at the cut point.
This process was done in different ways, each with its advantages, disadvantages and specific applications. This process was called genetic engineering or genome engineering until the advent of CRISPR technology.
CRISPR technology relied on a cellular mechanism found in nature, discovered by scientist Jennifer Doudna, who suggested using the mechanism by which microorganisms' immunity against viruses works to do what she called programmed genome editing, i.e. modifying the genome in a precise and controlled manner.
After that, she collaborated with scientist Emanuela Carpenter to develop the "CRISPR-Cas9 system", which became more comprehensive and suitable for editing all genomes.
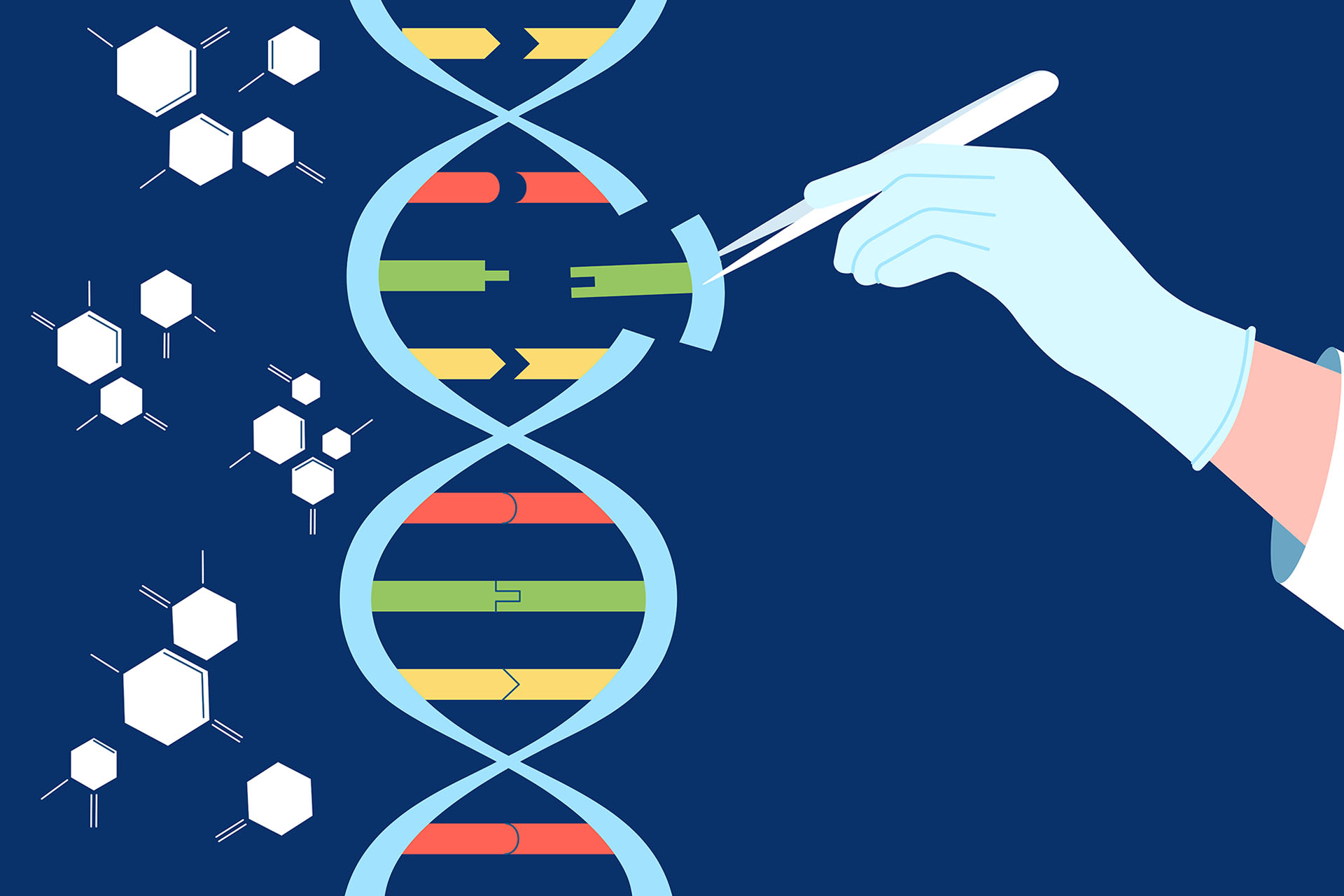
The system relies on two RNA molecules and a protein called Cas-9.
The system represents "molecular scissors" that cut the genetic material at a specific region, then insert a new sequence (sequence of genetic material) that is loaded with it and integrates it into the original genetic material of the organism.
Since the advent of this technology, its research and applied uses have multiplied, the most important of which was the emergence of treatments for genetic diseases that had no treatment before, including one of the genetic conditions that causes blindness due to a defect (mutation) in a specific gene that causes problems in the retina of the eye;
Scientists were able to prepare a drug that is injected into the eye once, to replace the defective gene with a healthy copy of the same gene, but the value of this treatment is close to one million dollars for the eyes.
CRISPR technology is not the first genome editing technology, but it made the process simple and affordable (Getty Images)
Edit single genetic bases
After the CRISPR technology was awarded the Nobel Prize, scientists became more interested in research and development in the mechanisms of action of the technology and the development of variations and applications for it, and funded research projects in the field of genome editing using this technology increased.
This led to the emergence of base editing technology, a technique based on CRISPR technology, but instead of replacing a long stretch of genetic material with another, it replaces only one nitrogenous base with another base, and it was developed by a team led by the scientist David Liu from Harvard University, and published in Nature in 2016.
In many cases, a change in one of these bases occurs as a result of an error in copying the genetic material during the process of cell division, and this change is called a mutation.
This mutation may be ineffective, given the length of the gene and the presence of many non-functional regions in it.
However, few of these mutations have a huge effect, which may lead to stopping the gene's work entirely or a major change in its function, if the mutation occurs at one of the functionally important points of the gene.
Single bases technology is mainly useful for correcting this type of error or making a new mutation to give a gene a new function or improve its performance.
The most common example of a single base mutation is an autosomal oncogenic mutation.
In the ICGC Cancer Genome Sequencing Project, scientists found more than 80 million mutations in the genomes of 2,500 cancer patients, most of which were of this type.
They also found that a small percentage of them lead to the disease or contribute to its development and increase its severity, while the vast majority of them do not contribute to the disease, but they often result from it due to the imbalance that affects the diseased cells.
The technology was used to treat acute lymphoblastic leukemia and to achieve complete recovery for the patient (British press)
Cancer treatment has no cure
Recently, scientists in the United Kingdom were able to use this modern technology to treat a case of acute lymphoblastic leukemia and achieve complete recovery for the patient, while there was no treatment for this type of cancer before the advent of this technology.
The case is of a 13-year-old girl named Elicia from the English city of Leicester, who was diagnosed in May 2021 with acute T-cell leukemia, which is also known as acute lymphoblastic leukemia, which is a blood cancer that occurs as a result of a defect in one of the two types of white blood cells. T-cells, resulting in a significant increase in the number of these dysfunctional cells until the color of the blood becomes white.
All attempts to treat the child with known methods, such as chemotherapy and bone marrow transplantation, failed to achieve tangible progress, until a team from the Great Ormond Street Hospital in London decided to try a treatment based on the base editing technique.
The team - made up of a group of scientists and doctors - modified the normal T cells 3 modifications using this technique.
The first modification stops T cells from attacking other cells and bodies completely.
While the second modification removes a certain compound from them, which is a compound characteristic of this type of cell, and finally the last modification protects these cells from death by chemotherapy.
The new cells were finally modified to attack and kill every T-cell (normal or abnormal) in the child's body;
This is done by recognizing the compound characteristic of T cells, and killing any cell that contains it.
As a result of this treatment, the new cells eliminated "all" T-cells in the blood, and then a new marrow was transplanted to the child, which produced healthy T-cells.
By the end of 2022, 6 months have passed since this operation, during which the disease did not appear in the child again, although the doctors had decided that she would be under observation for another period to monitor the success of the new treatment.
The promising technology holds many applications in basic science, health, and therapy, but it takes more time for such applications to become widely available and affordable.