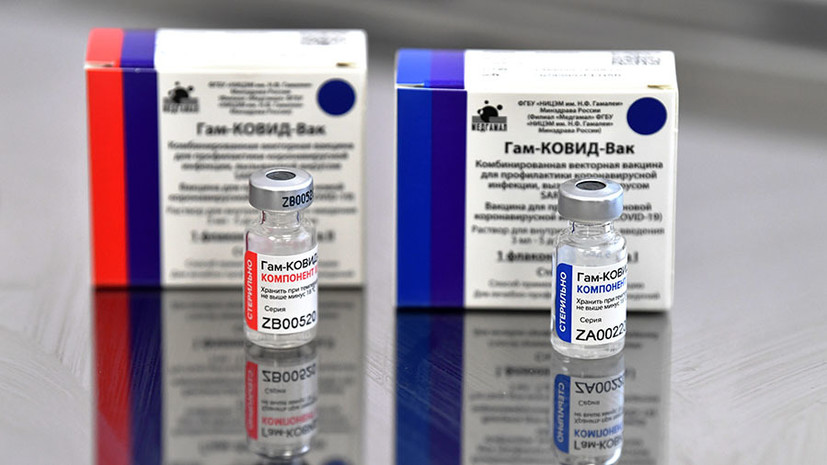
“At WHO we have a [procedure for inclusion in] the so-called register of funds for use in emergencies.
We are requesting documentary evidence of the safety and efficacy of [the drug], as well as documentation of the manufacturing process, ”said Harris.
According to her, it often takes a long time to check technological processes to "find out all the details, conduct an inspection and so on, so that the vaccine will be given the green light."
“The same procedure is followed by other regulators.
So this can cause delays, ”she said.
Earlier, the organization's official representative, Tarik Yazarevich, said that WHO will inspect the production of the Sputnik V vaccine no earlier than January 2022.