An immunology professor said that people who refuse to take the second dose of the AstraZeneca vaccine for fear of reports that it has caused rare clots may receive the J&J vaccine.
He attributed this to the proximity of its formula to the AstraZeneca vaccine, in addition to a group of other vaccines whose composition is the same as the vaccine.
This came in response to the wide scientific debate that was raised regarding the possibility of switching Corona vaccines from one dose to another.
In an interview with the evening program on Al-Jazeera Mubasher, Professor Talal Nsouli, a spokesperson for the American College of Asthma and Immunology, said that the AstraZeneca vaccine was given to 25 million people, of whom 86 suffered a stroke, which is a small percentage, he said, explaining that among the recipients of the vaccine are infected persons in The basis for other diseases.
Europe is studying cases of blood clots due to the "Johnson & Johnson" vaccine
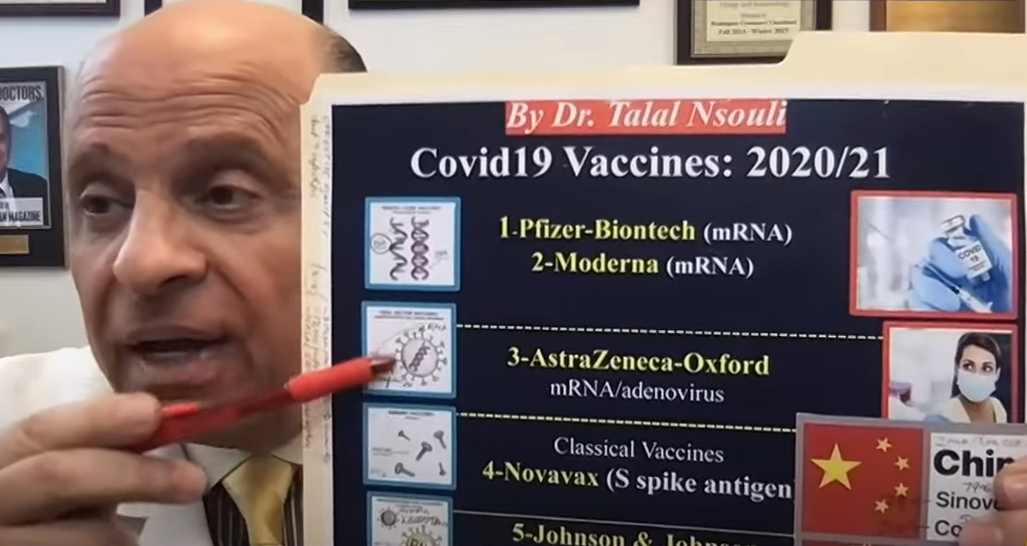
Dr..
Talal Nasouli, spokesperson for the American College of Asthma and Immunology, answers @ m3alhakim pic.twitter.com/mu3q9Yv1nw
- Al Jazeera Mubasher (@ajmubasher) April 9, 2021
Nsouli stressed the need to give the vaccine until the target rates of between 60% and 70% are reached to achieve herd immunity to stop the spread of the virus.
He said that vaccination rates are currently moving slowly in the world, which poses a difficulty in fighting the virus and stopping its spread, stressing that returning to normal life and stopping the economic decline consists in taking vaccinations to stop the spread of the virus.
The professor of immunology praised the efforts of Dr. Al-Arabi Moncef Al-Salawi, in charge of vaccinations in the United States, who contributed to revitalizing the vaccination campaign there and achieving goals that US President Joe Biden had said he wanted to achieve in the first 3 months of his presidency.
He said that most vaccines have effectiveness in fighting new mutations of the virus, stressing the need for regular vaccination campaigns in all countries of the world if herd immunity is to be reached to stop the spread of the virus.
With regard to giving vaccines to younger age groups, he stressed the need for that and said that many companies have conducted experiments to administer vaccines to different age groups that have proven great effectiveness, and that there are experiments now being conducted on age groups that include young children.
Should children be vaccinated against Coronavirus?
pic.twitter.com/IrzFpBI8e5
- Al Jazeera Mubasher (@ajmubasher) April 9, 2021
No recommendation for cross-vaccination
On the other hand, the World Health Organization said that it did not recommend what is called "cross-vaccination" against Corona disease, which uses two doses of different vaccines to complete immunization from the disease.
Margaret Harris, WHO spokeswoman, told German news agency DPA that there was not yet enough information on the potential risks of a first dose of the AstraZeneca vaccine and a second dose of another vaccine.
Margaret Harris referred to a preliminary recommendation by a WHO expert panel in February, and according to this recommendation, the same product should be injected for the two vaccination doses at the moment, at a time when experts called for more research to verify the use of a mixture of vaccines.
Extensive scientific controversy regarding the possibility of switching # Corona vaccines from one dose to another
Dr..
Talal Nasouli, spokesperson for the American College of Asthma and Immunology, answers @ m3alhakim pic.twitter.com/g0g62Wdwc9
- Al Jazeera Mubasher (@ajmubasher) April 9, 2021
In Germany, there is pressure to use such a cross-vaccination, as people under the age of 60 can no longer receive the AstraZeneca vaccine due to its risk of causing blood clots.
According to the German Minister of Health, 2.2 million citizens under the age of 60 have already received a primary vaccination with the AstraZeneca vaccine, but the question now is how to obtain full protection by vaccination, as this requires them to receive a second dose.
Eugen Brisch, a member of the Board of Directors of the German Foundation for Patient Protection, said that "without sufficient data on the potential risks, a second vaccination should not be given with another vaccine."
The Standing Committee on Vaccination in Germany recommends that these people now receive a second vaccination with the Pfizer / Biontek or Moderna vaccine, after 12 weeks, but no decision has yet been made.