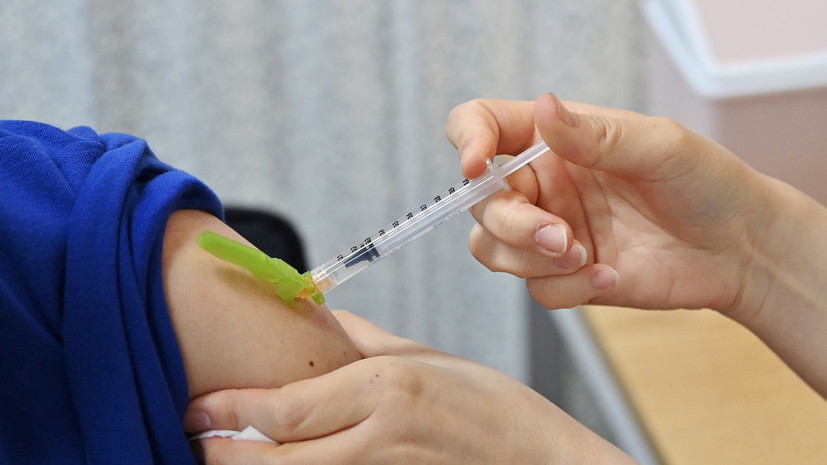
“Together with our partner BioNTech, we administered the dose to the first child participants in the ongoing Phase 1/2/3 global study,” a company spokesman said on n-tv.
He added that the aim of the clinical trials is to evaluate the safety, tolerability and efficacy of the vaccine in children aged six months to 11 years.
“We are proud to be doing this much-needed research for children and families looking forward to a possible vaccination,” said a Pfizer employee.
Almost 4.5 thousand children are expected to take part in the tests.
Pfizer expects the drug can be used in this age group from the beginning of next year.
Earlier, the American company Moderna announced the launch of clinical trials of its own vaccine against coronavirus infection in children.