What is the likelihood of a stroke in those who received the AstraZeneca-Oxford vaccine against the emerging corona virus (Covid-19)? What is the latest information about the vaccine? And after the vaccine is suspended in some countries, what should you do if you receive the first dose? And why did Germany specifically suspend the vaccine?
First, we will calculate the probability of a stroke in those who received the "AstraZeneca-Oxford" vaccine, then compare it with the probability of stroke in the normal population, and we confirm here that these numbers are general and for the purpose of comparison, and they are not a substitute for following the directions of the health authorities in your country and the doctor.
How many strokes have been recorded in those who received the AstraZeneca-Oxford vaccine?
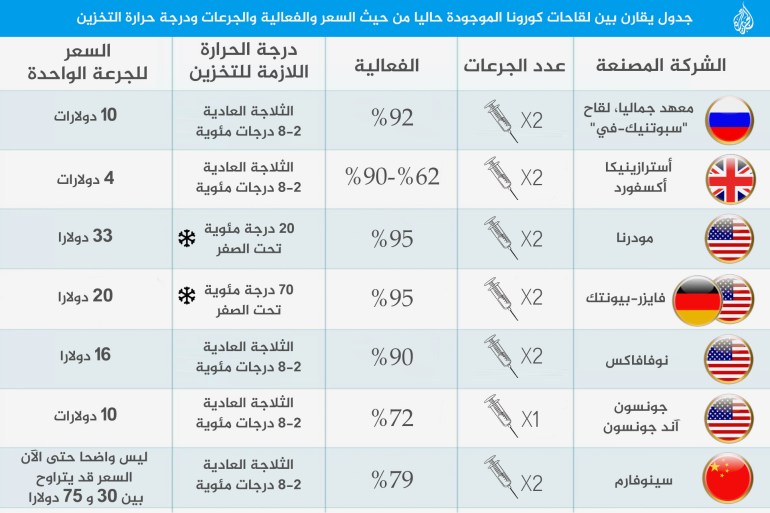
37 blood clots are among the more than 17 million people who have been vaccinated in the European Union and Britain.
Among them were 15 cases of "deep vein thrombosis" and 22 cases of "pulmonary embolisms), from January 4 to March 14, the date of the statement issued by AstraZeneca;
That is, 70 days.
If we do a simple and general calculation, the rate of occurrence of a blood clot over a year (365 days) per million over a year is 11.3 strokes per million people.
We calculated this by counting the number of strokes that may occur over a year (instead of 70 days), and then dividing it by 17;
Let's get the number 11.3 strokes per million people over a year.
We confirm here that this is a general calculation and estimation, based on the available data for the moment.
What is the likelihood of strokes in the general population?
The likelihood of deep vein thrombosis and pulmonary embolism is approximately 1 per thousand per year in the adult population;
That's about a thousand in every million adults, year-round.
And according to medical sources and studies.
What does this mean?
According to previous figures, those who received the "AstraZeneca-Oxford" vaccine are less likely to have strokes compared to the general population.
"About 17 million people in the European Union and the United Kingdom have now received our vaccine, and the number of blood clots reported in this group is less than the hundreds of cases expected in the general population," said Ann Taylor, AstraZaineca Chief Medical Officer.
The European Medicines Agency says the number of blood clots reported in vaccinated people is no higher than the number observed in the general population.
What did the World Health Organization say?
Today, Wednesday, a World Health Organization (WHO) vaccine safety committee said that it believes that the benefits that accrue from using the AstraZeneca vaccine to prevent Covid-19 outweigh the risks, and has recommended continuing immunization with it.
The World Health Organization said that the Global Vaccine Safety Advice Committee is carefully examining the latest available data on the safety of the "AstraZeneca" vaccine.
"Once the review is completed, the World Health Organization will publish the results immediately," the World Health Organization said in a statement a day after its experts held a closed meeting.
"Until now, the World Health Organization (WHO) considers the benefits of using the AstraZeneca vaccine to outweigh the risks, and recommends that immunization campaigns continue."
For its part, the European Medicines Agency said it was investigating reports of 30 cases of unusual blood function disorders out of 5 million who received the vaccine.
A total of 45 million doses of AstraZeneca were distributed across the region.
The European Agency, the regulatory body concerned with licensing medicines and vaccines, is scheduled to announce its findings on Thursday.
But its president, Emir Koc, said she saw no reason to change the recommendation regarding the "AstraZeneca" vaccine, one of the four vaccines that the agency has approved to use so far to prevent the disease.
Confused
However, with the previous figures, there is something puzzling about a very rare type of stroke. When the first comment was announced last week, the questions were related to a possible link between the vaccine and the formation of blood clots or clots, such as phlebitis, for example, or even a pulmonary embolism. .
However, on Monday, the Paul Ehrlich Institute of Medicine, which advises the German government, went further, saying that "by analyzing new data, we see a large accumulation of a specific type of cerebral thrombosis, which is very rare, associated with a lack of platelets." These are the factors that in turn prompted Germany to suspend the "AstraZeneca" vaccine as a precaution, followed by France and Italy.
Odile Lunay, an infectious disease expert and member of the Covid-19 vaccination committee, set up by the French government, told AFP that "cerebral vein thrombosis" is on the one hand much rarer than conventional thrombosis, and on the other hand it is likely To be more severe, these rare clots can cause strokes (cerebral vascular accidents).
On the other hand, several countries have reported hemorrhagic cases that can coincide with “diffuse intravascular coagulation events,” Professor Lunay added, “These are very exceptional syndromes, and they can appear in the context of serious infections, leading to clots and bleeding at the same time.”
At this stage, there is no evidence of a link between the vaccine and these events, and this point will be clarified by the European Medicines Agency, which will publish its findings Thursday.
How can the risk be determined?
Professor Lunay asserts that "the question is whether these few events are higher than the incidence rate, which we usually record" in the population in the absence of the vaccine.
Germany reported 7 cases of strokes in cerebral vessels, out of more than 1.6 million injections, on Monday.
German Health Minister Jens Young said that "the risk is very low," adding, "But if these cases are related to the vaccine, it will be a higher than average risk."
The ministry stated, Tuesday, that out of 1.6 million people, it is expected statistically that "about 1 to 1.4 cases" will be recorded, not 7, of cerebral vascular thrombosis.
On the contrary, the European Medicines Agency repeated, Monday, in a statement that "the number of stroke-related events in people who have received the vaccine does not appear to be higher than in the general population."
But even if their number is small, it is the atypical character of these events, which have been recorded, that baffles the specialists.
Professor Philippe Nguyen, a specialist in thrombolysis at the French Society of Hematology, told the French press, "We remain in an exceptional case, and therefore these files must be effectively examined."
To see if there is a link with the vaccine.
On the other hand, it was found that "Covid-19" itself could cause blood clots. Professor Stephen Evans (London School of Hygiene and Tropical Medicine), as quoted by the British organization "Science Media Center", says that "some of the coagulation problems that have been observed currently may be Caused by Covid-19, not the vaccine. "
For his part, Paul Hunter, a professor of medicine at the University of East Anglia, told BMJ medical journal that the infection mortality rate for men in their mid-forties due to Covid-19 is 0.1%, or about a thousand deaths per million Injury, which is much greater than the potential for "cerebral vein thrombosis".
He added, "It is clear that this potential link needs a comprehensive investigation, but we need to look at the real harm caused by the delay in vaccination campaigns at a time when the rate of Covid-19 infection is still on the increase in many European countries."
Which are the most benefits or risks?
As with all medicines, the primary question is how to strike a balance between the benefits and risks of the vaccine.
That is, a determination of the extent to which the benefits of the vaccine outweigh the disadvantages.
"We remain fully convinced that the benefits of the AstraZeneca vaccine in preventing Covid-19 infection and the associated risks of hospitalization and death outweigh the risks of these side effects," said European Medicines Agency director Emer Cook, during a press conference.
The Belgian Minister of Health, Frank Vandenbroek, in contrast to a number of his European counterparts, stressed that "stopping this vaccination campaign, knowing that there is such a spread of the virus, would be an irresponsible act because it is a sure and effective protection against this disease." From specialists across Europe.
After the vaccine has been suspended in some countries, what should you do if you receive your first dose of AstraZeneca-Oxford?
In a report, the French magazine Le Nouvel Observateur says that the first dose of AstraZeneca - like other vaccines - stimulates an immune response that makes it possible to reduce dangerous forms of Covid-19.
According to data from Public Health England, a single dose of the "AstraZeneca" or "Pfizer-BioNTech" vaccine reduces the likelihood of a hospital stay by more than 80%.
However, the data indicate that the effectiveness of the vaccine in reducing severe cases diminishes if the second dose is not obtained. The Supreme Health Authority in France recommended, on March 2 this year, to wait 12 weeks to obtain the second dose of the "AstraZeneca" vaccine, on In light of what the data of the third phase of clinical trials showed.
Can I get a second dose from another vaccine?
The magazine says that the answer is not known at present, and the University of Oxford announced, on February 4, that it would launch a study to obtain answers in this regard.
It is expected that this pilot study, which is the first of its kind in the world, will include 820 volunteers over the age of 50 years, and will test the results of mixing doses of the vaccines "Fire-Biontech" and "AstraZeneca".
Jonathan Van Tamm, Deputy Chief Medical Officer of England, stressed the importance of "having data that can support a more flexible vaccination program", especially in light of the shortage of supplies.
According to the British expert, "It is possible that the immune response would be better, if the vaccines were mixed, with an increase in the concentration of antibodies in the blood serum for longer periods."