China News Service, January 6, reported that the number of confirmed cases of the new crown in the United States continues to increase, medical institutions in many places are overwhelmed, and medical resources are facing exhaustion.
Due to the poor progress of vaccination, the White House is considering halving the dose of the new crown vaccine developed by Modena in order to speed up the vaccination.
In this regard, vaccine manufacturers and US regulators have expressed doubts.
According to data from Johns Hopkins University in the United States, as of 6:22 on the 6th Beijing time, the cumulative number of confirmed cases of new crown in the United States exceeded 21 million, and the number of deaths exceeded 356,000.
On December 29, local time, people in Washington, the US capital, ate in an outdoor "bubble" restaurant.
Recently, due to the rapid increase in the number of new coronary pneumonia cases, Washington announced the closure of all indoor restaurants, and only outdoor restaurants can continue to operate.
Intensive care unit in Midoland becomes saturated
Los Angeles has a very low chance of survival being given up for treatment
After the traditional holiday in the United States, the number of new coronavirus infection cases continued to rise in many states, including California, Florida, North Dakota and other states, where the intensive care unit was almost saturated.
On January 5, local time, the Arizona Department of Health Services (ADHS) reported that as of the 4th, only 8% of intensive care beds in the state were available, and only 8% of conventional hospital beds were available.
In California, where the epidemic is severe, the number of new crown infections in Los Angeles County rose from about 400,000 on November 30 to 800,000 on January 2.
The surge in cases caused the capacity of the intensive care unit in the entire region to plummet to zero.
Due to the lack of available hospital beds, the Los Angeles County Emergency Medical Services (EMS) instructed paramedics not to transport patients with a small chance of survival to the hospital, and to save the use of oxygen, causing public concern and condemnation.
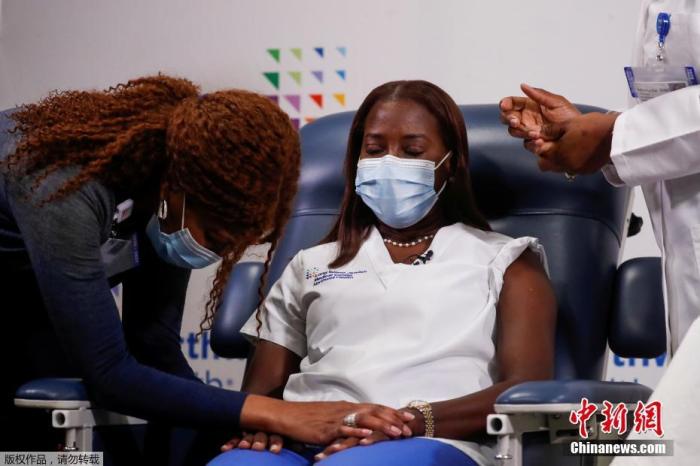
On January 4, 2021 local time, New York, USA, nurse Sandra Lindsay received the second shot of the new crown virus vaccine at the Long Island Jewish Hospital.
The White House may halve the vaccine dose to speed up vaccination
FDA: No basis yet, it's too early
At the same time, the progress of the US new crown vaccination work is not smooth.
The original goal of the United States was to vaccinate 20 million people by the end of 2020, but according to the Centers for Disease Control and Prevention (CDC) figures, the federal government has only distributed more than 15 million doses of vaccine to the United States so far, and only 4.5 million doses have been vaccinated. It accounts for only 30% of the issued dose.
Slavi, the head of the US Federal Vaccine Program "Action Curvature Speed", said on the 3rd that the US government is considering halving the dose of the new crown vaccine developed by Modena in order to speed up the vaccination rate of the people.
In response, the U.S. Food and Drug Administration (FDA) stated that these changes are "premature" and any unauthorized dosage changes will seriously endanger public health.
However, the "New York Times" stated that the National Institutes of Health (NIH) and Modena, a subsidiary of the US Department of Health, are analyzing vaccine research data to see if the vaccine dose can be halved and the vaccine supply accelerated.
On the other hand, the Chairman of the WHO Strategic Advisory Group on Immunization (SAGE), Claveto, stated on the 5th that SAGE’s recommendation is to vaccinate two doses of Pfizer-BioNTech vaccine within 21 to 28 days.
However, in order to allow more people at higher risk of infection to be vaccinated, he believes that countries can extend the interval between the two doses of vaccine.
In this regard, the German biotechnology company BioNTech said that the 95% protection data of the new crown vaccine jointly developed by them and Pfizer is based on 2 injections and 21 days apart.
BioNTech emphasized: "The safety and effectiveness of different vaccination intervals have not been evaluated."