↑The staff demonstrated the vaccine.
Photo by Xinhua News Agency reporter Luo Xin
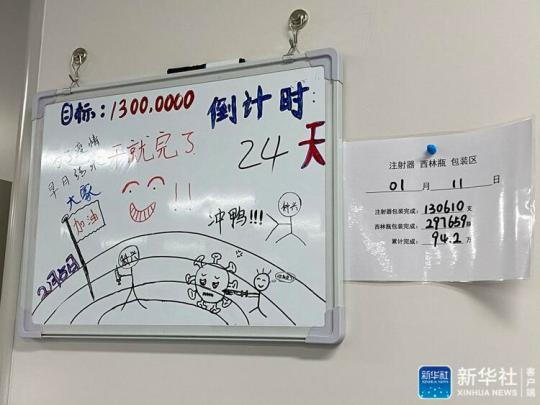
Xinhua News Agency, Beijing, January 17 (Reporter Luo Xin) "For an early end of the epidemic, everyone! Come on! Do it! Duck!" The new coronavirus in Beijing Kexing Zhongwei Biotechnology Co., Ltd. in the southern suburbs of Beijing In the inactivated vaccine packaging workshop, such encouraging words were written on a small whiteboard.
On the small whiteboard, there is also a cartoon of a small person holding a needle to inject the new crown virus, and next to it are the workshop's production goals and countdown.
On several production lines running at high speed, the staff are orderly carrying out the final packaging of vaccine production.
"Since we started production here at the end of August 2020, machines have been operating almost 24 hours a day and workers have been working in three shifts, just to ensure domestic vaccine supply demand and fulfill China's new crown vaccine commitment as a global public product." Kexing Holding Biotechnology Co., Ltd. Chairman Yin Weidong said.
At a press conference held by the Joint Prevention and Control Mechanism of the State Council on January 13, Wang Bin, Inspector of the CDC of the National Health and Medical Commission, introduced that China will start vaccinations against the new coronavirus for key populations on December 15, 2020. The current doses are It has exceeded 10 million.
Overall, the country’s new crown virus vaccination is stable and orderly, and the new crown vaccination work will gradually be promoted from key populations to the general population.
According to Liu Peicheng, Director of Brand and Public Relations of Kexing Holding Biotechnology Co., Ltd., from cell culture to packaging, the production cycle of a vaccine is 48 days at the fastest, and it will go through 6 major steps.
Because it is a rolling production, after the production line is running, finished products can be output basically every day.
At present, the domestic packaging line produces about 400,000 doses of finished vaccines in a single-dose package every day.
↑A small white board in the vaccine packaging workshop.
Photo by Xinhua News Agency reporter Luo Xin
According to reports, in January 2020, the Ministry of Science and Technology launched the first batch of emergency research projects, focusing on supporting 5 technical routes and multiple new crown vaccine research and development tasks.
In accordance with China's five vaccine technology routes, Kexing focuses on the research and development of inactivated vaccines.
In January 2020, the development of the new crown inactivated vaccine was started, the pre-clinical research was completed in April, and the clinical research phase was approved. Phase I and II clinical trials were carried out in China. In June, the vaccine was approved for emergency use in China. , Ran out of "Beijing Speed".
The reporter saw that a lot of advanced manufacturing equipment was used in the workshop site and automated operation was used to minimize direct contact between people and drugs.
According to reports, the first step in vaccine production is to produce the stock solution. Through the processes of cell culture, virus culture, inactivation, detection, and purification, a highly pure vaccine stock solution is obtained.
Then, the vaccine stock solution enters the preparation workshop.
"Kexing's current annual production capacity of the first-phase stock solution has reached 500 million doses, and the second-phase production line will be put into production in February this year, and the annual production capacity of the stock solution will reach 1 billion doses." Liu Peicheng said.
In the preparation workshop, the vaccine stock solution will be mixed with semi-finished products, filling and packaging.
"According to the operating specifications, the stock solution needs to be mixed with a certain proportion of inorganic salt auxiliary materials and aluminum adjuvants to form a semi-finished product. In this process, in order not to disturb the operating environment of the machine during the proportioning, we will require the staff to reduce unnecessary walking. ." Liu Peicheng said.
The vaccine semi-finished product made by the proportioning then enters the filling workshop, and then undergoes a light inspection to observe whether the filling quantity meets the requirements, whether there are scratches on the bottle wall, and whether the vaccine contains foreign objects.
In addition to the various tests on the production line, strict manual random inspections are also carried out in the laboratory.
↑The staff in the vaccine packaging workshop.
Photo by Xinhua News Agency reporter Luo Xin
"The stability of Kexing vaccine is very good. According to the results of accelerated thermal stability experiments, the new crown vaccine of Kexingkeleifu was placed at 25 degrees Celsius for 42 days, and at 37 degrees Celsius for 21 days, all indicators still meet the quality. It can be stored for a very long time under the condition of 2 degrees Celsius to 8 degrees Celsius. The current tentative validity period is three years." Liu Peicheng said.
This provides favorable conditions for the storage and transportation of vaccines.
Kexing uses cold-chain trucks to transport vaccines to CDCs in China, and uses temperature-controlled boxes to transport vaccines to all parts of the world by plane. At the same time, it uses temperature testers to monitor and record the entire process to ensure the safety and effectiveness of vaccines.
"After the vaccine arrives, the vaccine recipient can use specific software to read the data to verify whether the vaccine has indeed been maintained at 2 to 8 degrees Celsius." Liu Peicheng said.
Recently, Kexing shipped semi-finished products equivalent to 15 million doses of vaccine to Indonesia.
“At present, some of the new crown vaccines produced by Kexing are exported to countries that have vaccine research and development cooperation with China, such as Brazil, Turkey, Indonesia, etc.” Liu Peicheng said, in order to further expand the packaging capacity, Kexing and some cooperative countries have adopted export In the form of semi-finished products, filling and packaging lines are added overseas to increase global supply capacity.