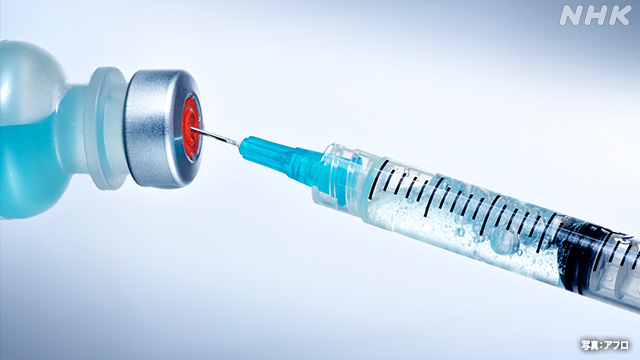
Vaccination of the new coronavirus has already begun in the United States and the United Kingdom, but in Japan as well, an application for approval of the vaccine of the American pharmaceutical giant Pfizer has been submitted, and it will be concluded whether it will be approved by the end of February at the earliest. It is a prospect.
In clinical trials conducted by Pfizer in the United States and Brazil, the vaccine has a protective effect of 95%, and in response to these results, the world's first vaccine was approved in the United Kingdom on the 2nd of this month, and vaccination began on the 8th. In the United States, an emergency use permit has been issued and vaccination has started on the 14th.
On the other hand, some of the people who were vaccinated in the United Kingdom and the United States had symptoms such as severe allergic reaction "anaphylaxis", so the CDC = Disease Control Center in the United States has allergic reactions to the ingredients contained in the vaccine. Those who have experience of showing are instructed not to inoculate.
In addition, the vaccine of the American pharmaceutical company Moderna also showed 94.1% efficacy in clinical trials, so the US FDA = Food and Drug Administration issued a permit for emergency use, and vaccination has started from the 21st. ..
What is the new "mRNA vaccine"?
All of these vaccines are completely new types of vaccines called "mRNA vaccines", which are not the virus itself, but the substance "mRNA" that transmits the genetic information of the virus, which is artificially created and administered by injection. I will.
By administering a vaccine, an antibody corresponding to the new coronavirus is produced by the action of immunity, and when the virus invades the body, the antibody attacks and prevents infection.
Inoculation in Japan When did you start?
Pfizer is also applying for approval in Japan on the 18th of this month, and is aiming to apply "special approval" that greatly simplifies the examination.
Based also data of clinical trials being conducted in the country, at the prospect that conclusion whether it is also approved in February as early as leaves, medical personnel Ministry of Health, Labor and Welfare is in prospect the end of February next year, a late March prospect to ensure the system to begin the vaccination of the elderly, our policy is to carry out the vaccination in preference, such as in people who are then underlying disease.
What is the current status of domestic vaccine development?
Vaccine development for the new coronavirus is also underway in Japan, and two domestic companies have begun clinical trials to confirm the safety of the vaccine by actually administering it to humans.
Of these, AnGes MG, a bio-venture company in Osaka, started clinical trials in June, the earliest domestic vaccine, and from this month it has increased the number of subjects to 500 and is continuing clinical trials.
This company is developing a kind of vaccine "DNA vaccine" that uses genes instead of the virus itself, and it is a mechanism to make antibodies that attack the virus in the body by administering it.
Shionogi Pharmaceutical Co., Ltd., a major pharmaceutical company headquartered in Osaka, began clinical trials on 214 people on the 16th of this month.
We are developing a type called "recombinant protein vaccine", which uses genetic recombination technology to artificially produce and administer only a part of the viral protein to produce antibodies in the body.
Is it difficult to confirm the effect of domestic vaccines?
However, clinical trials conducted in Japan have problems, the number of infected people is smaller than in Europe, the United States and South America, and the possibility of infection for those who participated in clinical trials is lower than in other countries, so the effectiveness of the vaccine will be confirmed. It has been pointed out that it is difficult.
In addition, when vaccines from overseas manufacturers are widely inoculated in Japan in the future, the number of infected people will decrease further, and many people will approach the state of so-called "herd immunity" where they have immunity. It has been pointed out that it may be more difficult to confirm the preventive effect.
For this reason, PMDA (Pharmaceuticals and Medical Devices Agency), which reviews drugs in Japan, has the option of conducting large-scale clinical trials overseas after completing initial clinical trials for a small number of people in Japan. It is said to be one.