- Technology: Graphene and other supermaterials to make the future
- Interview. Pablo Jarillo-Herrero: "MIT gave me a lot of money and said: 'Do what you want with it'"
- Neuroscience: Graphene implants in the brain to treat epilepsy and stroke
- Tomás Palacios. 'The mobile phone will disappear, it will be integrated into our clothes'
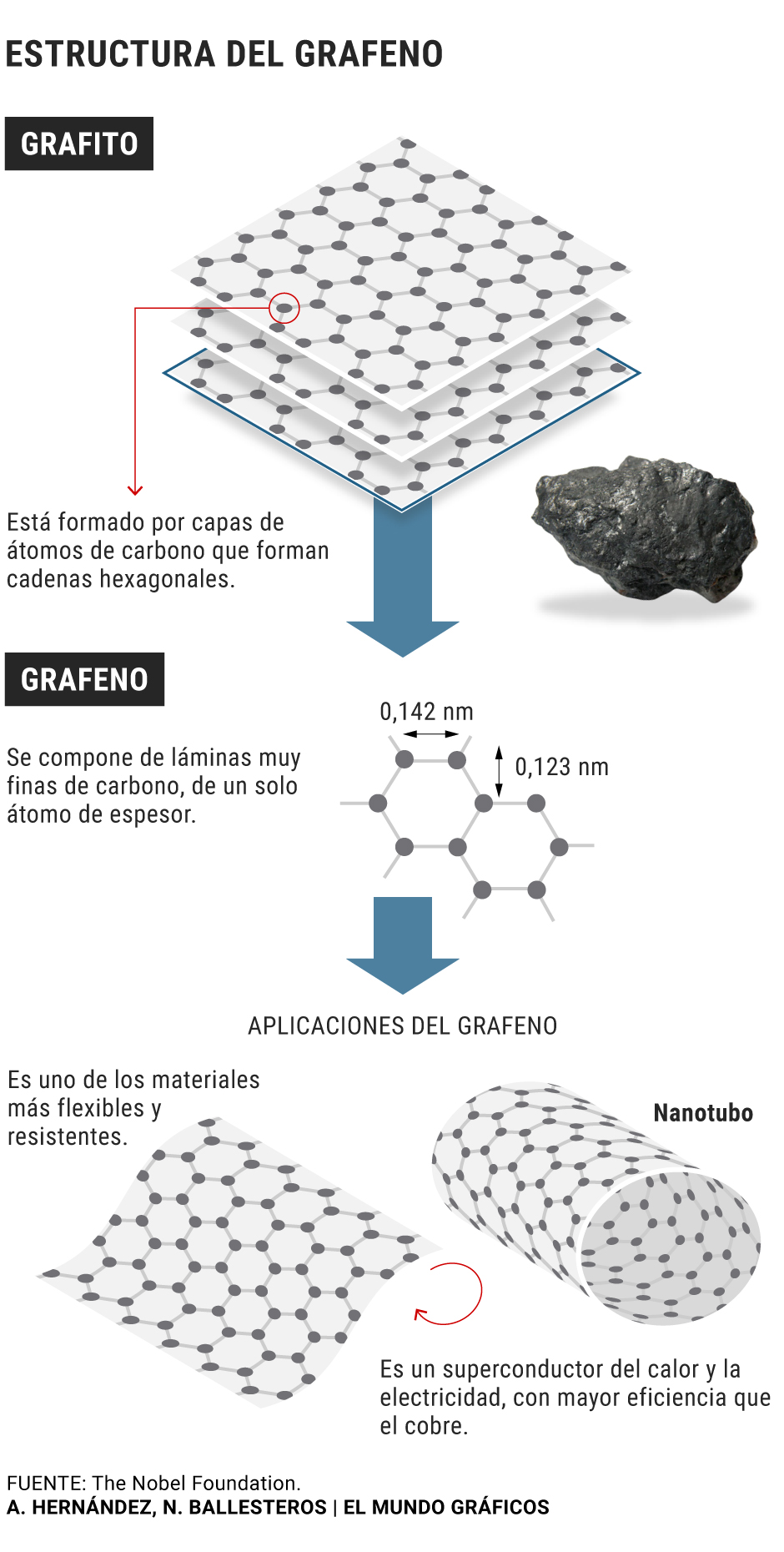
15 years ago two researchers from the University of Manchester, Andre Geim and Konstantin Novoselov, used cellophane strips to separate layers of carbon only one atom thick from pieces of graphite. That ultra-fine carbon, graphene, proved to be flexible, a good conductor of heat and electricity, lighter than paper and two hundred times stronger than steel.
The finding earned them the Nobel Prize in 2010 and revealed to the world the existence of a new material for which scientists imagined hundreds of potential applications. But after a first stage of study and cataloging, research around graphene began to stagnate. Since then, practical uses are taking a long time, largely because one of its main attractions is its hardness also makes it a difficult material to work.
However, throughout this last year graphene has returned to take center stage. In 2018, the Spanish team Pablo Jarillo-Herrero at the Massachusetts Institute of Technology (MIT) discovered that combining two sheets of this material, tilted at a small angle, led to a new range of unknown behaviors, thanks to changes in their electrical properties.
Thus began a completely new line of research, baptized by its creators as twistronic, since it is based on giving a twist or twisting the structure of atoms. Now, scientists from the University College of London (UCL) have managed to materialize these findings by creating a new folding device capable of storing energy; a supercapacitor that can bend up to 180º without losing performance and that after 5,000 charge cycles retains 97.8% of its capacity.
The supercapacitor has a size of 6 cm and is formed by two identical electrodes, located on opposite sides of a gel that acts as a chemical means to transfer the electric charge. In this case the authors have used it to successfully illuminate dozens of LED lights. The work, described Monday in the journal Nature Energy solves a recurring problem in the manufacture of batteries: the difficulty of storing large amounts of energy in small spaces. "We have started with materials that give our supercapacitor a high power density (which determines how fast it can load or unload) but also a high energy density (which determines the operating time)," explains the first author of the study , Zhuangnan Li. "Normally, only one of those characteristics is achieved, achieving both is a crucial advance."
Collaboration between disciplines
A team of chemists, engineers and physicists has collaborated to conceive that new design. They manufactured the electrodes by combining several layers of graphene to create a dense structure, but at the same time porous, capable of trapping ions of different sizes. " Imagine you only need ten minutes to fully charge your electric car or two for your phone and it lasts all day, " says Professor Ivan Parkin, responsible for the investigation.
"We have shown that this system charges very quickly, that we can control the [energy] output and that it has excellent resistance and flexibility, which makes it ideal for miniaturized electronics and electric vehicles." Thanks to twistronics, scientists aspire to achieve the ability to induce different types of behavior for graphene, manipulating its structure and properties, but for that they still have to understand the physics behind their superconductive activity.
Although it is still a prototype, this work shows the enormous potential as a portable power source for smartphones or smart fabrics. In addition, it does not use liquid electrolytes, which minimizes any risk of explosion .
However, its creators recognize that before being a competitive alternative in the market there are still important obstacles to save. " The modified graphene we use is not yet available on a commercial scale , " says Parkin, "so for flexible electronic devices, it could take even a few years to market, even more for cars." However, the authors also remember that research around graphene is again one of the most intense, so it is likely that the next few years will bring new advances. "So far we have reached a new understanding about how to fit the size of the ions used to transport the charge with the layers of the supercapacitor, something that will allow us to better store energy," adds Parkin.
It is precisely this unique design that maximizes energy density up to a record 88.1 watt-hours per liter (Wh / L), the highest figure recorded by a carbon-based supercapacitor. Compared to the traditional lead and acid batteries of slow and long duration, used in electric vehicles, they have a similar value, 50-90 Wh / L. But in this case the difference is that the supercapacitor developed by the UCL team has twice the power density, more than 10,000 watts per liter.
According to the criteria of The Trust Project
Know more- Science and Health
- science
- Graphics
Meteorology Why has the "historic" storm of Gloria been so devastating?
Graphic report Australia: zero zone of climate change
Biotechnology Nanorevolution: the era of microscopic robots that will cure diseases is coming